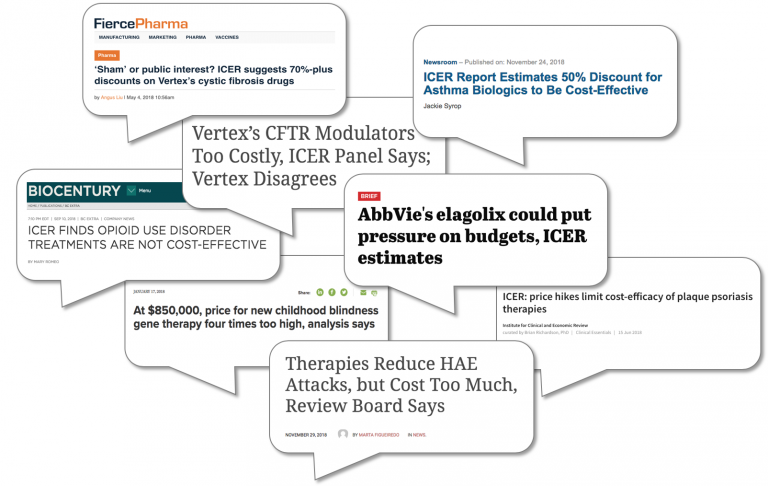
97% of Evaluated Drugs are Overpriced, According to ICER
2018 was not a good year for biopharma manufacturers, based on the Institute for Clinical and Economic Review’s (ICER) evaluation of interventions across various disease areas. Seemingly every month, ICER generated negative press for the biopharma industry. Headlines read:
Do Research Groups Align on the Value of an Intervention? A Prelude to our ISPOR Barcelona Presentation
Do Research Groups Align on the Value of an Intervention? A Prelude to our ISPOR Barcelona Presentation By Matthew Sussman
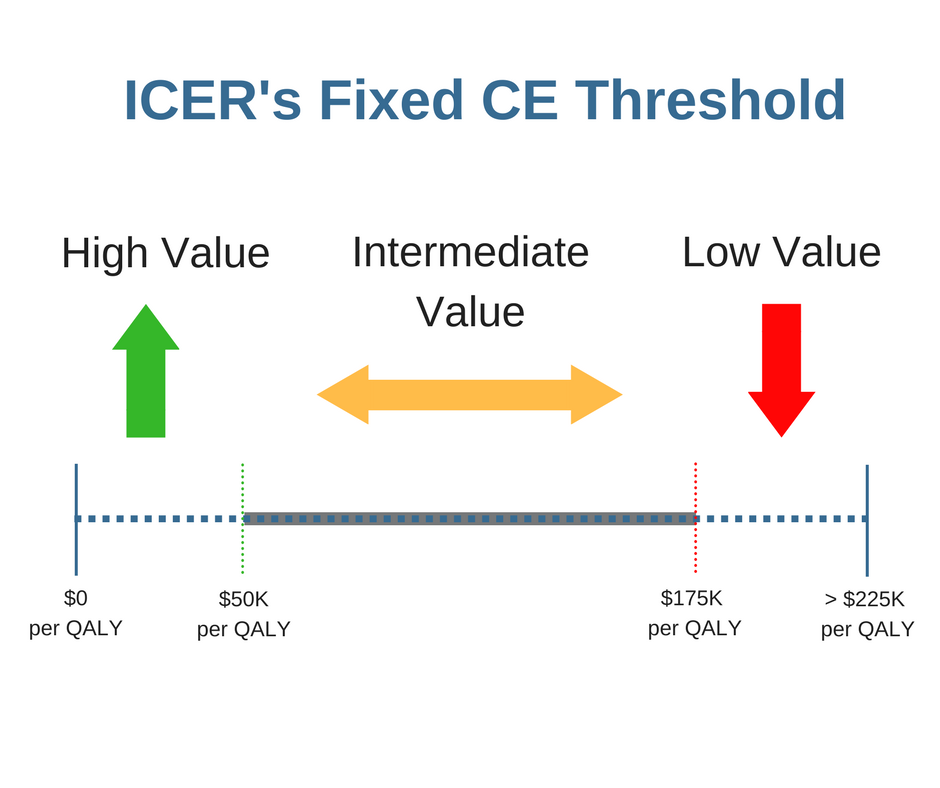
Do Fixed Cost-Effectiveness Thresholds or CE Threshold Magnitudes Matter?
In my last post, I highlighted three subtle differences between ICER’s and NICE’s methodologies when conducting cost-effectiveness (CE) analyses. These differences, if overlooked, could actually lead to very different recommendations for coverage, reimbursement, and pricing. Two of these important differences revolve around CE thresholds,[…]
ICER vs. NICE: Three Key Differences in Approach and Appraisal
Back in December, I recapped an issues panel titled, “Should ICER be NICE (or Not)?” from ISPOR’s 20th Annual European Congress, which sought to compare the use of ICER’s value assessment framework versus NICE’s guidelines when conducting and interpreting cost-effectiveness (CE) analyses. In that post, I summarized the ideas expressed during the[…]

ICER Versus NICE: The Verdict From Our ISPOR Glasgow Issue Panel
A smashing success! ISPOR hosted its 20th Annual European Congress in Glasgow, Scotland last month, boasting nearly 5,000 attendees and satisfying our deepest sweet tooth cravings thanks to the lunch menu’s endless supply of teacake biscuits, caramel wafers, caramel shortbread, and IRN Bru. In addition, the conference hosted three well-attended[…]
Should ICER Be NICE (or Not)?: A Prelude to Our ISPOR Glasgow Issue Panel
ISPOR’s 20th Annual European Congress in Glasgow is fast approaching, with the first plenary session scheduled for this Monday, November 6th. I will be attending as part of BHE’s team and am looking forward to the plenary sessions discussing the evolution of value in healthcare, including the increase in value-based care initiatives, the future of[…]
What You Should Know When Considering ICER’s Value Assessment Framework
Over the past several months, our Modeling & Evidence team has been busy tracking the Institute for Clinical and Economic Review’s (ICER) movements. In early February 2017, ICER released a revised Value Assessment Framework, the conceptual framework for guiding their evaluations of clinical and cost-effectiveness. After a three month open-comment[…]

A Need for Alignment: How ICER's New Cost-Effectiveness Framework Compares with the Second Panel's Recommendations
Methodologists in the HEOR community have been busy over the past 9 months. In September 2016, the Second Panel on Cost-Effectiveness in Health and Medicine published a special communication in JAMA (and subsequent hardcover book) that sought to provide guidance for improving the quality of cost-effectiveness (CE) analyses. A few months later in[…]

How Does ICER’s Incremental Cost-Effectiveness Analysis Stack Up to Industry Practice?
In early February, the Institute for Clinical and Economic Review (ICER) presented proposed updates to its Value Assessment Framework, based largely on stakeholder feedback received during a public comment period in late 2016. These updates focused on four domains of the framework: (1) comparative clinical effectiveness; (2) incremental[…]
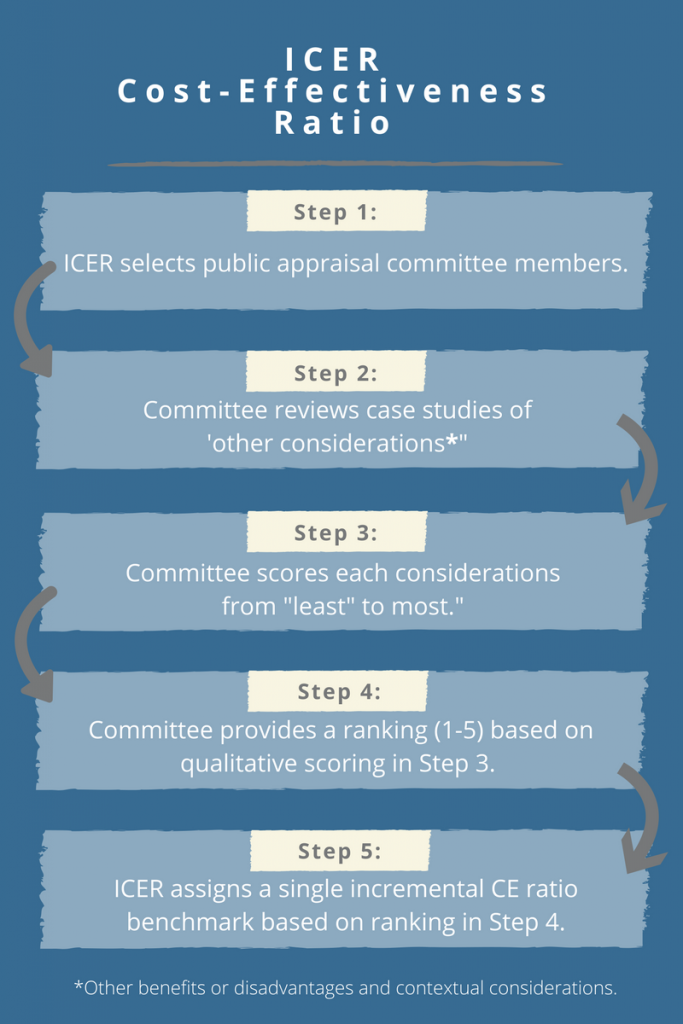
ICER's New Cost-Effectiveness Threshold Raises 5 Major Questions
Early last month the Institute for Clinical and Economic Review (ICER) released a revised Value Assessment Framework for public comment based on feedback received from patients, clinicians, life science companies, and other stakeholders. While a majority of the proposed changes lacked real luster, there is one proposed change that pharma should[…]
